american academy of pediatrics covid vaccine 12-15
Food and Drug Administration granted an Emergency Use Authorization for use of the vaccine in the 12- to 15-year age group. Pfizer-BioNTech COVID-19 vaccine expires 12 months after manufacturing if stored frozen at ultra-cold temperatures -90C to -60C -130F to -76F.

As School Resumes Uf Health Pediatrics Expert Gives Advice On Covid 19 Safety Uf Health University Of Florida Health
Pfizer was approved for use in this age group in January 2022 and Moderna was approved for use in ages 6 -17 in June 2022.

. The FDAs COVID-19 vaccine updates on Monday also included changing the time interval for a booster. Can only receive the COVID-19 mRNA vaccine. Children 511 will get a 10-microgram dose compared to the 30-microgram dose that adults and children 12 and older receive.
In this age group 41 of the cases were reported as community. COVID-19 is now one of the top 10 causes of death among adolescents ages 12 to 17 said Dr. This booster dose will provide optimized protection against COVID-19 and the Omicron variant About 86 million adolescents ages 12-15 years half of this age group are fully vaccinated according to the CDC.
NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. Nucleic acids found naturally in all our cells instructs them to make protein. Adolescents moved a step closer to being able to receive a COVID-19 vaccine after officials on Monday approved Pfizer-BioNTechs vaccine for those ages 12-15 years.
Cases among Americas youth. The number of children ages 12 17 who have tested positive for COVID-19 is 13. Adults who received a Johnson.
The AAP strongly recommends all eligible adolescents to be vaccinated as soon as possible. For example if the manufacture date is March 2022 322 include March as one of the 12 months. Pfizer and BioNTech conducted trials in more than 2000 adolescents ages 12-15 with half randomized to receive the vaccine and half to receive a placebo.
Encourage your child to keep doing their part to protect others. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age. People are considered up to date if they have received all recommended doses and boosters for their age.
1 the Pfizer-BioNTech COVID-19 Vaccine product authorized for children ages 511 years or 2 the Pfizer-BioNTech COVID. On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th. The American Academy of Pediatrics AAP is urging the US.
The Pfizer-BioNTech COVID-19 vaccine was approved by the CDC in persons aged 16 years on December 12 2020 following an EUA from the FDA on December 11th. The rollout of the pfizerbiontech coronavirus vaccine for children ages 12 to 15 in the united states has been reminiscent of when those very first covid-19 vaccine doses were administered late. 31 2021- The American Academy of Pediatrics AAP has launched a multi-faceted national marketing campaign encouraging parents to get their children ages 12 and older vaccinated against COVID-19.
Sara Oliver co-lead for the ACIP COVID-19 Vaccines Work Group. In a new policy statement published today the American Academy of Pediatrics AAP recommends vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine. Trials showed the Pfizer-BioNTech vaccine was 100 effective and presented no serious safety concerns in adolescents leading the Food and Drug Administration FDA to grant emergency.
Children who will turn from age 11 years to 12 years. Right now children and adolescents 5 to under 18 in the US. The Centers for Disease Control and Prevention have announced their emergency use authorization for Pfizer-BioNTechs COVID-19 vaccine for use in adolescents ages 12-15 years old a pivotal step in increasing eligibility for the vaccine in the United States.
The number of cases among this age group grew by 38 during March and April this year. To calculate the expiration date add 12 months to the manufacture date including the month of manufacture. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.
These doses can be given five months after the primary series and must use the Pfizer-BioNTech vaccine. The FDA issued an EUA for the Pfizer. COVID-19 mRNA vaccines contain messenger RNA mRNA which is made up of nucleic acids.
Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12. About 5 million of them are eligible for a booster. Food and Drug Administration highlighting that the Delta variant has created a new and pressing risk to children under age 12.
The American Academy of Pediatrics wrote a letter Thursday to the US. The AAP recommends against giving the vaccine to children under 12 until authorized by the FDA While the FDA is considering full approval of the vaccine for ages 12 through 15 it is available under emergency use authorization for this age group now and AAP strongly recommends all eligible adolescents be vaccinated as soon as possible. Earlier this week the US.
FDA authorization of the Pfizer-BioNTech COVID-19 Vaccine allows children who will turn from age 11 years to 12 years between their first and second dose in the primary series to receive for either dose. Both steps were part of a long-standing rigorous and transparent process that leads to. This approval comes after the Food and Drug Administrations emergency use sign-off.
The disease accounted for 13 of all. The Centers for Disease Control recommends the Pfizer-BioNTech and Moderna COVID-19 vaccines for kids ages 12 to 15. About 53 of adolescents ages 12-17 years are fully vaccinated according to an AAP analysis of CDC data.
The American Academy of Pediatrics AAP recommends that all eligible infants children and adults age 6 months and older should get the COVID-19 vaccine as soon as they can. People ages 12 and older who had a Pfizer-BioNTech primary series can get a booster at five months instead of six. The most common side effects in adolescents were pain at the injection site fatigue headache chills muscle pain fever and joint pain consistent with trials in older teens and adults.
Friday 28 May 2021 1548 The European Medicines Agency on Friday recommended that the use of the coronavirus vaccine made by Pfizer and BioNTech be expanded to children ages 12 to 15 a decision. While the FDA is still considering full approval of the COVID-19 vaccine for ages 12-15 it is still available under emergency use authorization for this age group.
Kids Covid Vaccine Side Effects What To Know

Covid 19 Moderna Begins Testing Its Vaccine In Children The New York Times
First Covid 19 Vaccine Authorized For Kids Ages 12 To 15 Smart News Smithsonian Magazine

Kids Covid Vaccine Twitter Chat Twitter

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr

Why Experts Say It S Vital That Parents Get Their Kids Vaccinated For Covid

Covid 19 Vaccine And Your Children Westmed

When Will Covid 19 Vaccines Be Available For Younger Kids Time
Pfizer Wants Authorization To Give Covid 19 Vaccine To Kids Over 12

Vaccinating Children Against Sars Cov 2 The Bmj
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News

Covid 19 Vaccine For Kids Benefits Are Many Here Are Answers To Parental Concerns Ask The Pediatrician Column Together Lancasteronline Com

Drugmakers Pfizer Covid 19 Vaccine Safe For Children Ages 5 11

Children 12 15 Years Old Can Now Get The Pfizer Covid 19 Vaccine Uconn Today
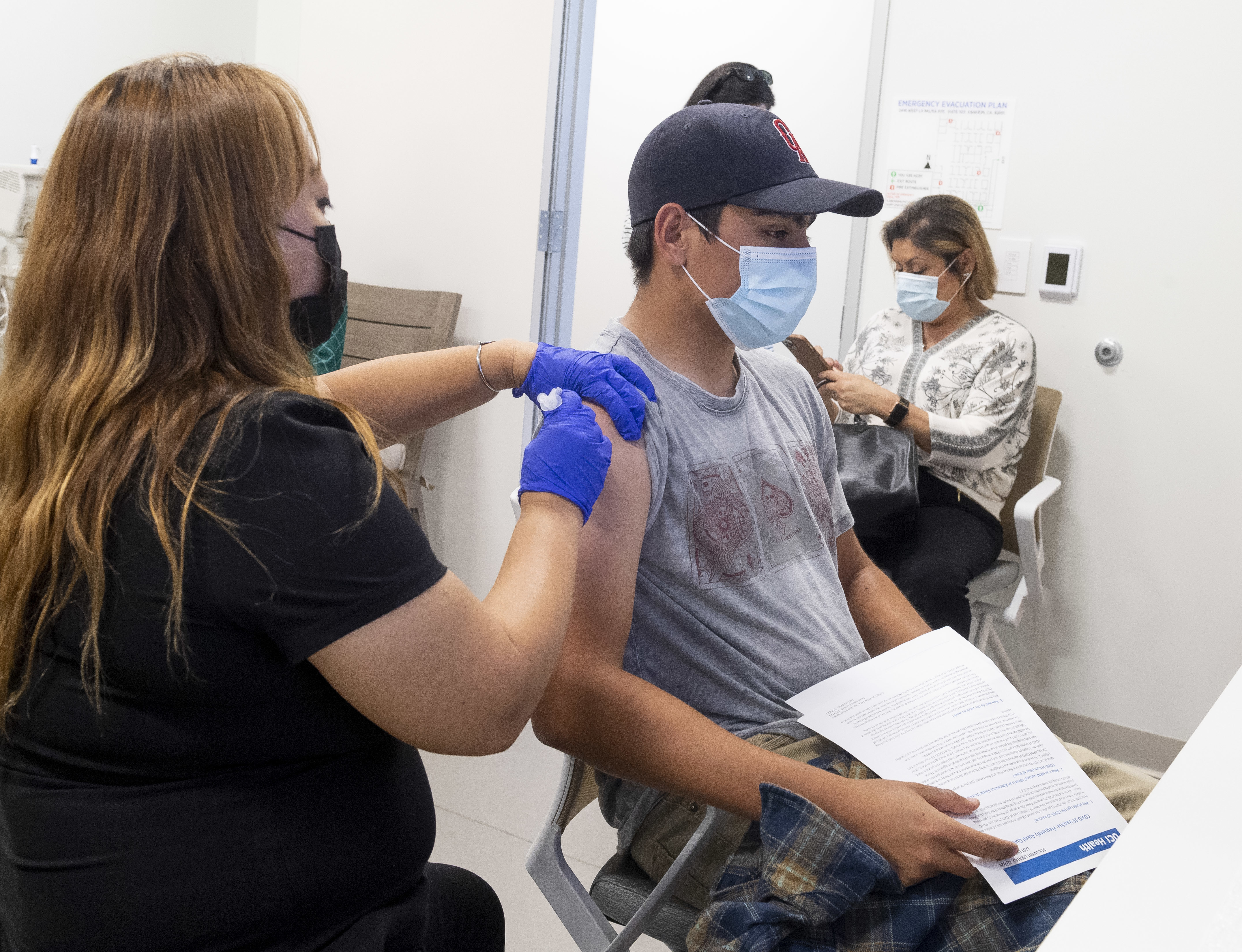
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

Covid 19 Vaccine For 12 To 15 Year Olds What Parents Should Know Methodist Health System Omaha Council Bluffs Fremont
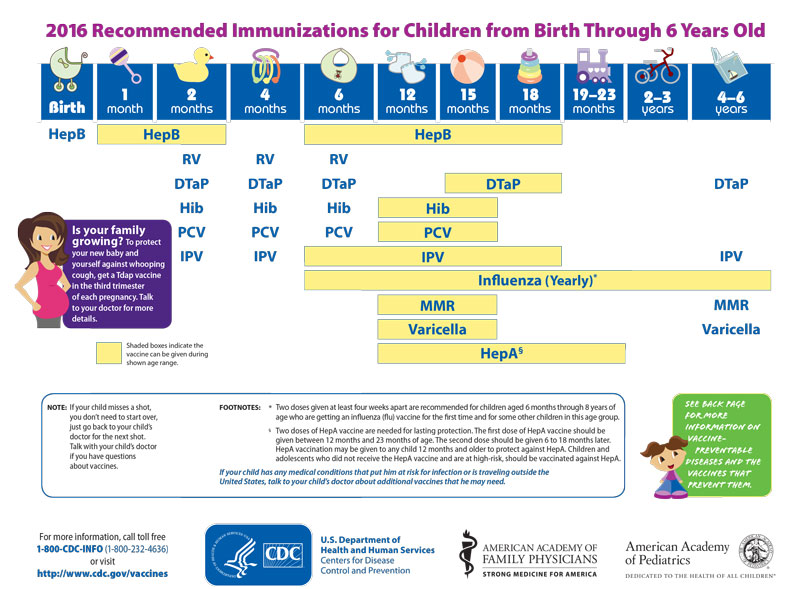
Immunization Schedule 0 5 Yrs Maryland Farms Pediatrics
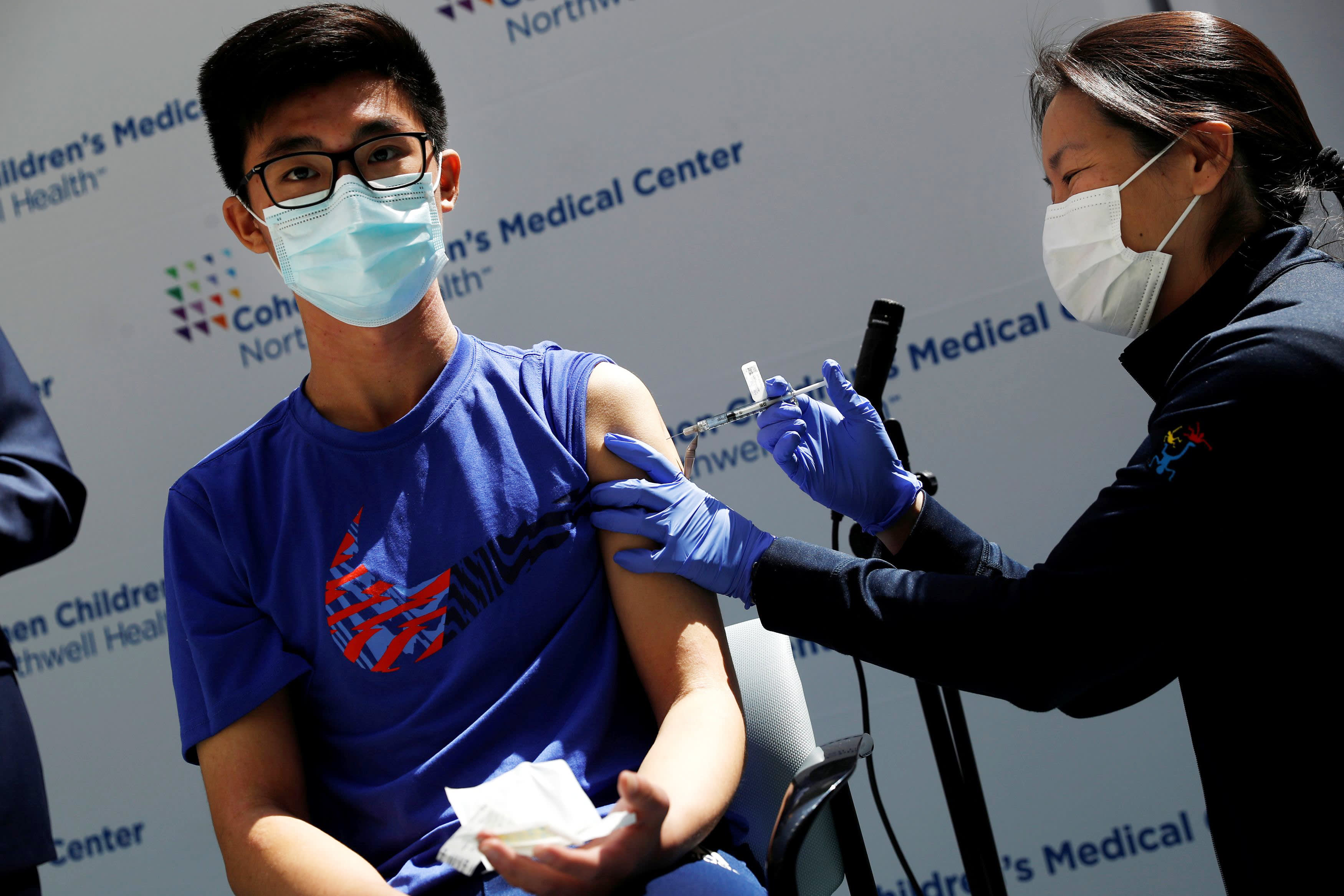
Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine